The Canadian Food and Drug Regulations (FDR) have established regulations for Food for Special Dietary use (FSDU) as well as food for infants, covered under Divisions 24 and 25 of the FDR. Health Canada is working towards modernizing these regulations to unlock potential for research, innovation, and flexibility within the regulations while keeping safety, nutrition, and support for these food categories top of mind.
Canada Gazette Part I has published a proposal for the regulatory modernization as well as a public regulatory consultation to comment. The consultation will be open for feedback until February 6, 2024.
1: Regulatory Reconstruction
The FDR is proposing a reconstruction of Divisions 24 and 25 to update and redefine product categories. The goal is to better and more simply categorize Foods for Special Dietary Purposes (FSDP) versus other foods in other regulatory categories. Health Canada included a schematic to illustrate the redefinition in the proposal, which is “Figure 1: Current regulatory and proposed modernized framework for Divisions 24 and 25, Food and Drug Regulations” as seen below:
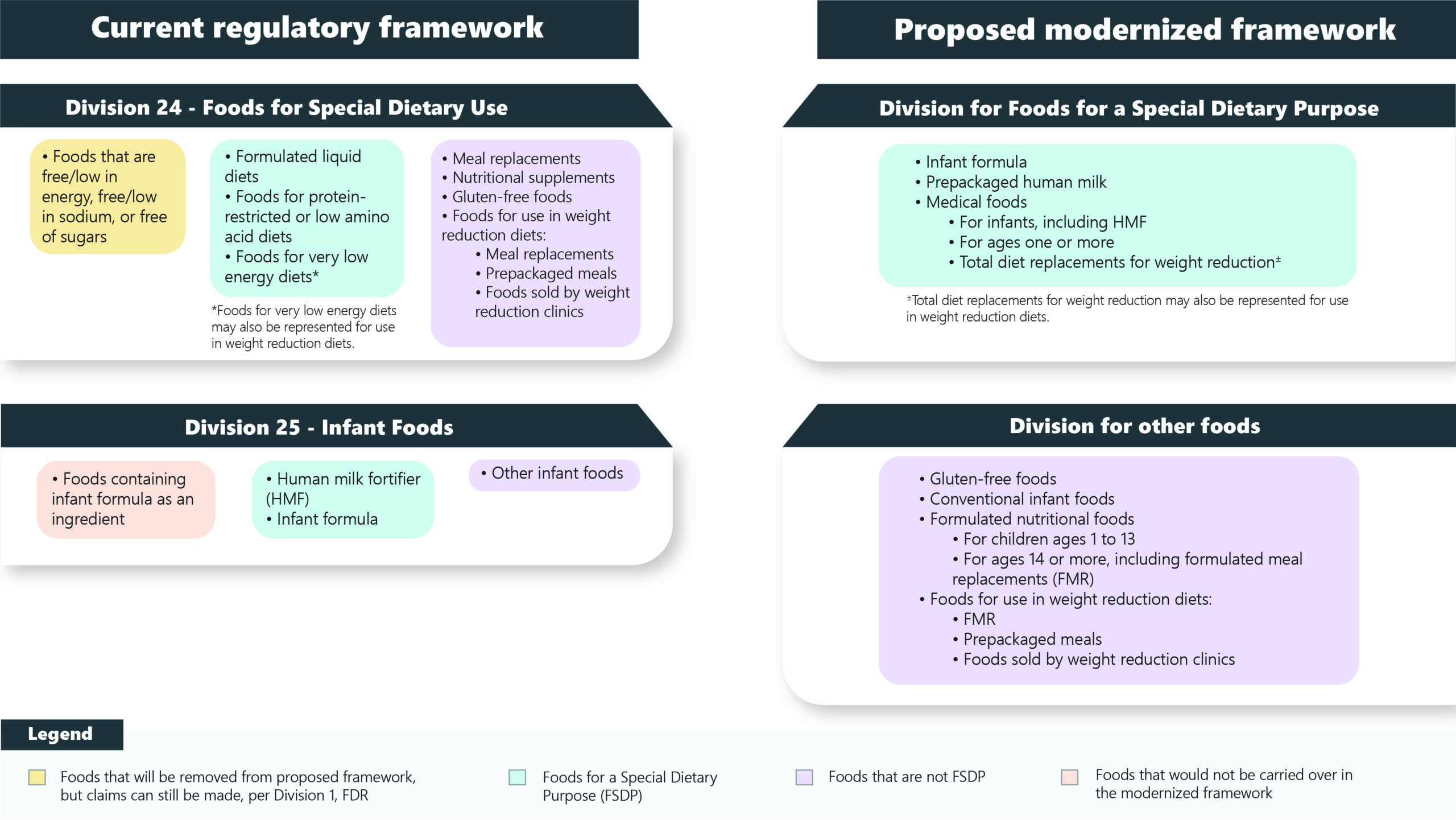
These updates propose the exclusion of foods that are only categorized as FSDP due to the use of a claim as well as foods containing infant formula.
2: Updated Definitions:
3: Food For Special Dietary Use Division:
A proposed new division that would encompass foods falling under the new definition of FSDU as well as products that are created for specific conditions and uses i.e., foods for dysphagia, foods for post-operative recovery, dyslipidemia, etc.
This division would also cover:
4: A Division for Foods That Are Not FSDP:
Health Canada is proposing a new division for food currently covered under Divisions 24 and 25 that do not meet the new definitions in the proposal. These foods include conventional infant food, gluten-free food, formulated nutrition food, and foods represented for use in weight reduction diets.
New definitions, compositional requirements, and labelling requirements are all outlined. Foods defined as not FSDP would be labelled with better alignment to conventional foods.
With such significant regulatory changes, it is encouraged to double-check your product categorization, as it may fit into a different regulatory pathway under the new definitions. Additional regulatory oversight and premarket approval may be needed in some cases. Changes to compositional and labelling requirements may mean an additional check will be needed in the coming year to stay compliant.
Stay Informed: to be well-prepared, we recommend regularly checking the Canada Gazette for ongoing updates with transitional periods and strategic planning.
Participate in Consultations: We recommend that you actively participate during the consultation and all ongoing consultation periods to share feedback, voice concerns, and contribute to the refinement of the new food compositional standards.
Get Expert Advice: Our consultants are here to assist you in reviewing your products and ingredients and provide you with a tailored compliance strategy to help you be prepared for the upcoming food compositional standards. Do not hesitate to get in touch today, and together, we can ensure your readiness for these critical changes.